
Mark S. Delaney
Associate Professor of Chemistry, McNeese State University,
Lake Charles, LA
Mark S. Delaney, Associate Professor (B. S. Chemistry California State University, Fullerton, 1975; Ph. D. Inorganic Chemistry, University of California, Los Angeles, 1980; Senior Research Chemist and Project Leader, Dow Chenmical Co., M. E. Pruitt Research Center (Central Research), 1980-1987; McNeese State University, 1987-present)
Information on Dr. Delaney's Spring 2008 classes
Dr. Delaney's Spring 2008 Schedule and Contact Info
Although I am an organometallic chemist, most of the projects I investigate have some relation to polymer chemistry. My research interests include anionic and Ziegler-Natta polymerizations, halatopolymers, permeabilities of gases through polymeric membranes and thermal characterizations of polymers. Short descriptions of these research areas follow:
ANIONIC POLYMERIZATIONS
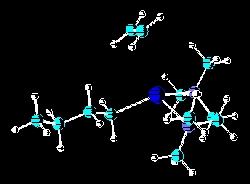 The anionic polymerization of ethylene via n-butyllithium chelated by various tertiary chelating diamines is one area of study. The most recent studies have been of the effects of steric hindrance of the active site by the alkyl substitutents in N,N,N',N'-tetraalkyl-1,2-ethanediamines and N,N,N',N'-tetraalkyl-1,3-propanediamines. Initial rates, yields, percent active sites have been areas of examination. Recently, these studies have been supplemented by molecular modeling studies. Related publications:
The anionic polymerization of ethylene via n-butyllithium chelated by various tertiary chelating diamines is one area of study. The most recent studies have been of the effects of steric hindrance of the active site by the alkyl substitutents in N,N,N',N'-tetraalkyl-1,2-ethanediamines and N,N,N',N'-tetraalkyl-1,3-propanediamines. Initial rates, yields, percent active sites have been areas of examination. Recently, these studies have been supplemented by molecular modeling studies. Related publications:
- M. S. Delaney, W. B. Marshall and J. L. Brewbaker, "The Rate of Ethylene Polymerization Initiated by Various Chelating Tertiary Diamine:n-Butyllithium Complxes," Journal of Applied Polymer Science, Vol. 42, 533-541 (1991).
- M. S. Delaney, W. B. Marshall and J. L. Brewbaker, "Initiator and Method for Polymerizing Ethylene and Preparing Block Copolymers Containing Ethylene," U. S. Patent 4,668,746 (1987).
- D. Boenig, G. Cook, L. Mayo, M. McGraw, W. G. Orloski, J. Pago, V. Victorian and M. S. Delaney, "Steric Effects of Chelating Tertiary Diamines on the Yields and Initial Rates of Anionic Ethylene Polymerizations Initiated by n-Butyllithium," Polymer Preprints, Vol. 36(1), 201-202 (1995).
ZIEGLER-NATTA POLYMERIZATIONS
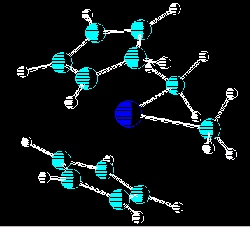 The most recent studies in this area been on the steric effects of the R groups on the amount of initiaton in the single site catalysts of the type bis(cyclopentadienyl)titaniumbis(R), where R may be chloro, methyl, phenyl, o-carboranyl and methyl-o-carboranyl. The cocatalyst used in these polymerizations was poly(methylaluminoxane) (MAO). As expected , the larger the R group, the lower the percentage of initiation in these single site catalysts. Also being currently investigated are the relationships of size and structure of MAO to the reactivity of ethylene polymerizations in which they are used as cocatalysts. New synthetic routes to MAO and modified MAOs are also being investigated. Related Publications:
The most recent studies in this area been on the steric effects of the R groups on the amount of initiaton in the single site catalysts of the type bis(cyclopentadienyl)titaniumbis(R), where R may be chloro, methyl, phenyl, o-carboranyl and methyl-o-carboranyl. The cocatalyst used in these polymerizations was poly(methylaluminoxane) (MAO). As expected , the larger the R group, the lower the percentage of initiation in these single site catalysts. Also being currently investigated are the relationships of size and structure of MAO to the reactivity of ethylene polymerizations in which they are used as cocatalysts. New synthetic routes to MAO and modified MAOs are also being investigated. Related Publications:
- W. M. Coleman, G. F. Schmidt, R. E. Campbell and M. S. Delaney "Heterogeneous Organometallic Catalysts Containing a Supported Titanium Compound and at Least One Other Supported Organometallic Compound," U. S. Patent 4,659, 685 (1987).
HALATOPOLYMERS
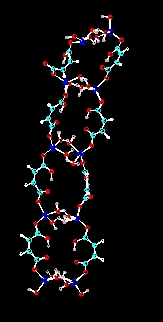 Halatopolymers are also known as metal salt coordination polymers. The systems currently under investigation are related to the fibrillar zinc hydrogen maleate halatopolymer reported by Vancso and Smercsanyi in 1981 (J. Polym. Sci. Polym. Phys. Ed., Vol. 19, 111-115 (1981), J. Polym. Sci. Polym. Chem. Ed., Vol. 20, 639-654 (1982)). Investigations have revealed that similar fibrillar halatopolymers may be synthesized containing Mn, Co and Fe. Additionally, related halatopolymers may be synthesized using phthalic and citraconic acids. Continuing investigation has suggested that the polymerization is actually related to a condensation-type mechanism. using this knowledge has led to the ability to synthesize fibrillar halatopolymers containing up to three different metals. The hope is to move toward a fibrillar halatopolymer which will function as a molecular magnet.
Halatopolymers are also known as metal salt coordination polymers. The systems currently under investigation are related to the fibrillar zinc hydrogen maleate halatopolymer reported by Vancso and Smercsanyi in 1981 (J. Polym. Sci. Polym. Phys. Ed., Vol. 19, 111-115 (1981), J. Polym. Sci. Polym. Chem. Ed., Vol. 20, 639-654 (1982)). Investigations have revealed that similar fibrillar halatopolymers may be synthesized containing Mn, Co and Fe. Additionally, related halatopolymers may be synthesized using phthalic and citraconic acids. Continuing investigation has suggested that the polymerization is actually related to a condensation-type mechanism. using this knowledge has led to the ability to synthesize fibrillar halatopolymers containing up to three different metals. The hope is to move toward a fibrillar halatopolymer which will function as a molecular magnet.
GAS PERMEABILITIES THROUGH POLYMERIC MEMBRANES
Studies in gas permeabilities include facilitated transport of oxygen, studies of free volume/density versus permeability relationships, permeabilities of modified poly(trimethylsilyl-1-propyne) membranes, and permeabilities of commercial of polymers (polyethylenes, ethyl/vinyl acetatecopolymers) Related articles:
- M. S. Delaney, D. Reddy and R. A. Wessling, "Oxygen/Nitrogen Transport in Glassy Polymers with Oxygen-Binding Pendent Groups," Journal of Membrane Science, Vol. 49, 15-36 (1990).
THERMAL CHARACTERIZATION OF POLYMERS
Studies include the development of methods to estimate the molecular weights of polyethylenes via the temperature of fusion as determined by differential scanning calorimetry. Another study relates the temperature of fusion of ethylene/vinyl acetate copolymers with the percentage of vinyl acetate in the copolymer. Related articles:
- C.-M. Feng and M. S. Delaney, "Estimates of Molecular Weights of Low Molecular Weight Linear Polyethylenes via Differential Scanning Calorimetry," Microchemical Journal, Vol. 48, 215-220 (1995).
CATIONIC POLYMERIZATIONS
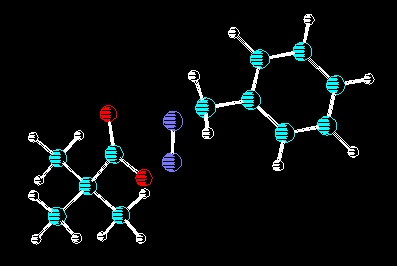 In conjunction with Dr. Ron W. Darbeau, there is an ongoing research
effort to use carbocations generated from deamination as initiators for
catonic polymerizations of various monomers. To view a poster on this
subject please click here.
In conjunction with Dr. Ron W. Darbeau, there is an ongoing research
effort to use carbocations generated from deamination as initiators for
catonic polymerizations of various monomers. To view a poster on this
subject please click here.
MOLeCULUAR MODELING STUDIES OF THE OLATION PROCESS
Along with Dr. Chris Rodriquez of the McNeese Chemistry Department, studies of the olation process of lithium monohydrate and eventually certain transition metal compounds (see halatopolymers above) are being conducted. the results of the molcular modeling studies are being compared with actual thermodynamic data of the olation process.
OTHER INTERESTS
My other intersests include fishing (especially flyfishing), flytying, fishing rod and reel repair, and music (I'm a mediocre piano player and play guitar poorly, but have a decent bass singing voice).
Mark S. Delaney
Associate Professor of Chemistry
Office: Kirkman 215 A
Lab: Kirkman 215
Office Phone: (337)475-5956
Lab Phone: (337)475-5956
E-mail: delaney@mail.mcneese.edu
or
chemprof2001@yahoo.com
Office Hours: MWF 10:00 - 10:50 am, MW(F) 1:00 - 3:00 pm, TTh 10:00 am - 1:00 pm
Occasionally on Fridays I may be in a faculty meeting and unavaialbe for afternoon office hours.
Other hours by appointment
Chemistry Office: Kirkman 225
Chemistry Office Phone: (337) 475-5776
Chemistry Office FAX: (337) 475-5950
Business Address:
Department of Chemistry
P.O. Box 90455
McNeese State University
Lake Charles, Louisiana 70609
Information on Dr. Delaney's Fall 2006 Classes
To the McNeese State University Webpage
To the McNeese Department of Chemistry Webpage (College of Science)
To the McNeese Department of Chemistry Maintained Webpage
To the Delaney Family Homepage
To Fishing in Southwest Louisiana
 The anionic polymerization of ethylene via n-butyllithium chelated by various tertiary chelating diamines is one area of study. The most recent studies have been of the effects of steric hindrance of the active site by the alkyl substitutents in N,N,N',N'-tetraalkyl-1,2-ethanediamines and N,N,N',N'-tetraalkyl-1,3-propanediamines. Initial rates, yields, percent active sites have been areas of examination. Recently, these studies have been supplemented by molecular modeling studies. Related publications:
The anionic polymerization of ethylene via n-butyllithium chelated by various tertiary chelating diamines is one area of study. The most recent studies have been of the effects of steric hindrance of the active site by the alkyl substitutents in N,N,N',N'-tetraalkyl-1,2-ethanediamines and N,N,N',N'-tetraalkyl-1,3-propanediamines. Initial rates, yields, percent active sites have been areas of examination. Recently, these studies have been supplemented by molecular modeling studies. Related publications:
 The most recent studies in this area been on the steric effects of the R groups on the amount of initiaton in the single site catalysts of the type bis(cyclopentadienyl)titaniumbis(R), where R may be chloro, methyl, phenyl, o-carboranyl and methyl-o-carboranyl. The cocatalyst used in these polymerizations was poly(methylaluminoxane) (MAO). As expected , the larger the R group, the lower the percentage of initiation in these single site catalysts. Also being currently investigated are the relationships of size and structure of MAO to the reactivity of ethylene polymerizations in which they are used as cocatalysts. New synthetic routes to MAO and modified MAOs are also being investigated. Related Publications:
The most recent studies in this area been on the steric effects of the R groups on the amount of initiaton in the single site catalysts of the type bis(cyclopentadienyl)titaniumbis(R), where R may be chloro, methyl, phenyl, o-carboranyl and methyl-o-carboranyl. The cocatalyst used in these polymerizations was poly(methylaluminoxane) (MAO). As expected , the larger the R group, the lower the percentage of initiation in these single site catalysts. Also being currently investigated are the relationships of size and structure of MAO to the reactivity of ethylene polymerizations in which they are used as cocatalysts. New synthetic routes to MAO and modified MAOs are also being investigated. Related Publications:  Halatopolymers are also known as metal salt coordination polymers. The systems currently under investigation are related to the fibrillar zinc hydrogen maleate halatopolymer reported by Vancso and Smercsanyi in 1981 (J. Polym. Sci. Polym. Phys. Ed., Vol. 19, 111-115 (1981), J. Polym. Sci. Polym. Chem. Ed., Vol. 20, 639-654 (1982)). Investigations have revealed that similar fibrillar halatopolymers may be synthesized containing Mn, Co and Fe. Additionally, related halatopolymers may be synthesized using phthalic and citraconic acids. Continuing investigation has suggested that the polymerization is actually related to a condensation-type mechanism. using this knowledge has led to the ability to synthesize fibrillar halatopolymers containing up to three different metals. The hope is to move toward a fibrillar halatopolymer which will function as a molecular magnet.
Halatopolymers are also known as metal salt coordination polymers. The systems currently under investigation are related to the fibrillar zinc hydrogen maleate halatopolymer reported by Vancso and Smercsanyi in 1981 (J. Polym. Sci. Polym. Phys. Ed., Vol. 19, 111-115 (1981), J. Polym. Sci. Polym. Chem. Ed., Vol. 20, 639-654 (1982)). Investigations have revealed that similar fibrillar halatopolymers may be synthesized containing Mn, Co and Fe. Additionally, related halatopolymers may be synthesized using phthalic and citraconic acids. Continuing investigation has suggested that the polymerization is actually related to a condensation-type mechanism. using this knowledge has led to the ability to synthesize fibrillar halatopolymers containing up to three different metals. The hope is to move toward a fibrillar halatopolymer which will function as a molecular magnet.  In conjunction with Dr. Ron W. Darbeau, there is an ongoing research
effort to use carbocations generated from deamination as initiators for
catonic polymerizations of various monomers. To view a poster on this
subject please click
In conjunction with Dr. Ron W. Darbeau, there is an ongoing research
effort to use carbocations generated from deamination as initiators for
catonic polymerizations of various monomers. To view a poster on this
subject please click