- ligand - an ion or molecule that coordinates to a metal atom or metal ion to form a complex.
- sigma-donor ligand - a ligand that acts as a Lewis base donating electrons from a lone pair to the metal center.
- pi-acceptor ligand - ligand that donates a pair of electrons from a lone pair to the metal center but also has the ability to accept electron density from the metal d orbitals into either d orbitals or pi-antibonding orbitals.
- chelating ligand - a ligand able to occupy two or more sites in the coordination sphere of a complex, usually donating two or more pairs of electrons to the metal center.
- monodentate - refers to a ligand that donates only one electron pair to a ligand, it is not a chelating ligand (agent)
- multidentate - refers to a ligand that donates more than one pair of electrons to a metal center, a chelating ligand (agent)
- bidentate - refers to a ligand that donates two pairs of electrons to a metal center, a chelating ligand (agent)
- coordination sphere isomerism - refers to structural isomerism of coordination complexes in which the lignds in the inner coordination sphere differ.
- linkage isomerism - refers to structural isomers of coordination compounds in which a ligand differs in its mode of attachment to a metal ion.
- structural isomerism - refers to compounds which have the same formula but having differing bonding arrangements of the atoms.
- stereosiomerism - refers to compounds having the same formula and bonding arrangement but differing in the spatial arrangements of the atoms (cis, trans, fac, mer, a, eq, and optical isomers)
- enantiomers - isomers which are non-superimposable mirror images of each other
- dextrorotary - describes and enantiomer which rotates plane polarized light to the right
- levorotary - describes an enantiomer which rotates plane polarized light to the left
- spectrochemical series - A series used in crystal field theory. A series going from low field ligands to high field ligands. Low field ligands result in high spin complexes while high field ligands result in low spin complexes. In general sigma -donor ligands are low field ligands while pi -acceptor ligands are high field ligands. An abbreviated spectrochemical series is given here:
Cl- < F- < H2O < NH3 < en < NO2- (N-bonded) < CN-
- crystal field theory - - a theory that accounts for the colors and magnetic and the other properties of transition metal complexes in terms of the splitting of the energies of the metal ion d orbitals by the electrostatic interaction with the ligands.
- crystal field theory - - a theory that accounts for the colors and magnetic and the other properties of transition metal complexes in terms of the splitting of the energies of the metal ion d orbitals by the electrostatic interaction with the ligands.
- RhCl3. 3 H2O
Rh3+, 18 electrons around Rh, name: triaquotrichlororhodium (III)

Co2+, 19 electrons around Co, name: hexacyanocobaltate (II) ion

Fe2+, 18 electrons around Fe, name: bromodicarbonylcyclopentaidenyliron (II)

Fe3+, 17 electrons around Fe, name: fac-triamminetrithiocyanatoiron (III)
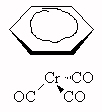
Cr0, 18 electrons around Cr, name: benzenetricarbonylchromium (0)

linkage isomerism, Fe3+ for both structures, 17 electrons around Fe for both structures, name: left: hexathiocyanatoferrate (III) ion, right: hexaisothiocyanatoferrate (III) ion

coordination sphere isomerism, Co2+ for each structure , 19 electrons around Co for each structure, name: left: hexaaquocobalte (II) chloride, right: tetraaquodichlorocobalt (II) dihydrate

optical isomerism, Ni2+ for each structure, 16 electrons around Ni for each structure, name: bromocarbonylchloro(triphenylphosphine)nickel (II), determination of R & S beyond scope of CHEM 102

cis-trans isomerism, Pt2+ for both structures, 16 electrons around Pt for both structures, name: left: cis-diamminedichloroplatinum (II), right: trans-diamminedichloroplatinum (II)

fac-mer isomerism, Fe3+ for both structures, 17 electrons around Fe in each structure, name: left: fac-triamminetribromoiron (III), right: mer-triamminetribromoiron (III)
Compounds are named in each example.
Compounds have electron counts indicated in each example.

8 electrons around Ti, Ti4+, name: tetrachlorotitanium (IV)

16 electrons around Ni, Ni2+, name: dichlorobis(triphenylphosphine)nickel (II)

18 electrons around Ti, Ti2+, name: dicarbonylbis(cyclopentadienyl)titanium (II)

18 electrons around Fe, Fe0, name: (eq)tricarbonyl(a)bis(triphenylphosphine)iron (0)