STUDY GUIDE FOR COORDINATION CHEMISTRY FOR CHEM 102
- Define:
- ligand
- sigma-donor ligand
- pi-acceptor ligand
- chelating ligand
- monodentate
- multidentate
- bidentate
- coordination sphere isomerism
- linkage isomerism
- structural isomerism
- stereosiomerism
- enantiomers
- dextrorotary
- levorotary
- spectrochemical series
- crystal field theory
- What is the oxidation state of the central atom in the compounds below?
- RhCl3. 3 H2O



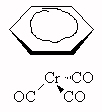
- What type of isomerism is demonstrated by the pairs of compounds
below?





- Name all the compounds on the study guide using IUPAC nomenclature
rules.
- How many electrons are around the central metal atom for all the drawn
structures on the study guide? (including those below)
















