Study Guide for CHEM 102 Coordination Cheimstry
- Define:
- cis
- trans
- fac
- mer
- ligand
- sigma-donor ligand
- pi-acceptor ligand
- chelating ligand
- monodentate
- multidentate
- bidentate
- coordination sphere isomerism
- linkage isomerism
- structural isomerism
- stereosiomerism
- enantiomers
- dextrorotary
- levorotary
- What is the solubity of Zn(OH)2 in 4.00 M NH3? KspZn(OH)2 = 2.1 x 10-16 and
KfZn(NH3)42+ = 2.9 x 109.
- What is the oxidation state of the central atom in the compounds below?
- RhCl3. 3 H2O



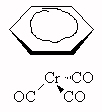
- What type of isomerism is demonstrated by the pairs of compounds below?





- Name all the compounds on the study guide using IUPAC nomenclature rules.
- How many electrons are around the central metal atom for all the drawn structures on the study guide? (including those below)




- Classify the ligands below as to whether they are i) anionic or neutral, ii) σ-donor or π-acceptor ligands, iii) monodentate or multidentate, and
iv) what number of electrons they donate to the metal center.



















