- FN2+, 3, dia
- F2, 1, dia
- C2, 2, para
- BBe, 0.5, para
- O2-, 1.5, para
- N2, 3, dia
- BF2-, 2, para
- CN-, 3, dia
- NO, 2.5, para
- CN-, 3, dia
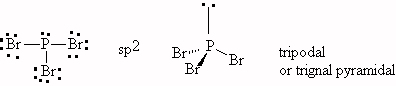
- PBr3, tripodal, sp3
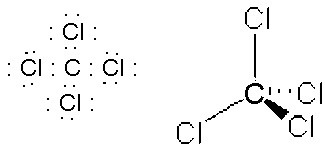
- CCl4, tetrahedral, sp3
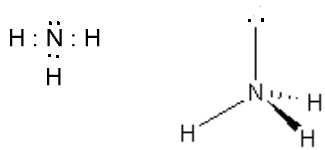
- NH3, tripodal, sp3
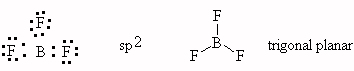
- BF3, trigonal planar, sp2
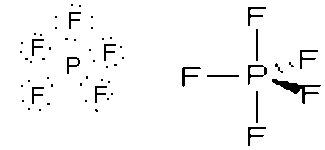
- PF5, trigonal bipyramid, sp3d
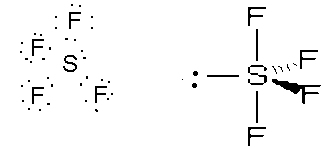
- SF4, wedge, sp3d
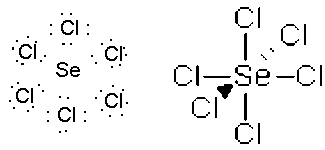
- SeCl6, octahedral, sp3d2
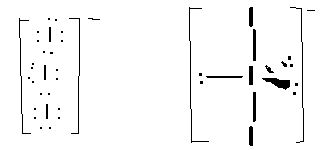
- I3-, linear, sp3d
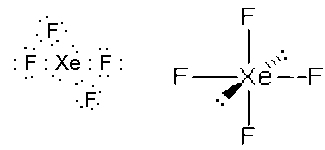
- XeF4, square planar, sp3d2
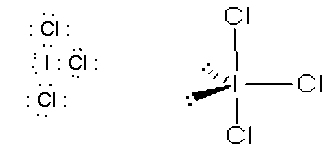
- ICl3, t-shaped, sp3d
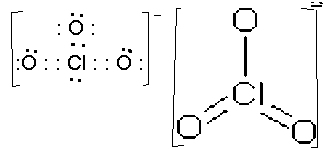
- ClO3-, trigonal planar, sp2 plus d orbital interactions in p bonds. Only one of three possible resonance structures is shown.
- MnSO4 manganese (II)sulfate
- AlPO4 alluminum phosphate
- P2O3 diphosphorus trioxide
- K3PO4 potassium phosphate
- KClO3 potassium chlorate
- Sc2S3 Scandium (III) sulfide
- N2O5 dinitrogen pentoxide
- Ca(NO3)2 calcium nitrate
- CoNO3 cobalt (I) nitrate
- FeSO4 iron (II) sulfate
- Define:
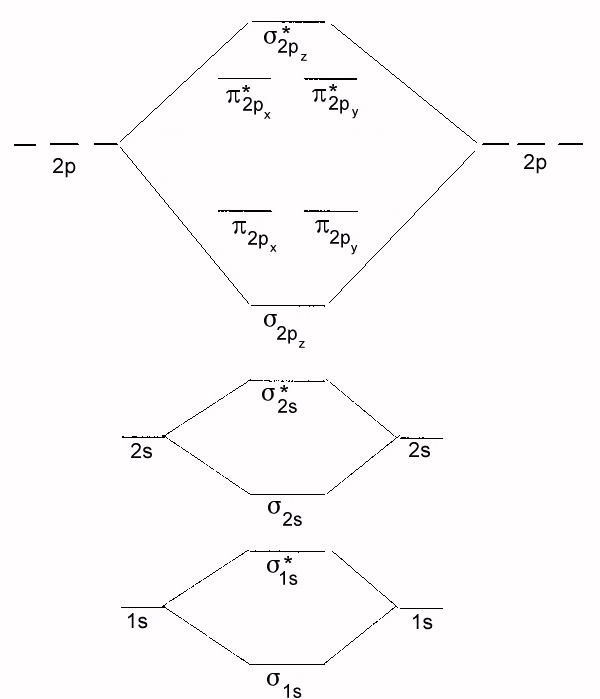
- bonding orbital-a molecular orbital which puts electron density between or above or below the potential bond between two atoms. It is designated by either no superscript or a superscript
- anti-bonding orbital-a molecular orbital that has no electron density between or above or below the axis of a potential bond between two atoms. It is designated with a superscript *
- molarity: Moles of solute per liter of solution, mol/L, abbreviated M.
- percent by mass: {(mass a)/(mass A + mass B +...)} x 100 = {(mass A)/(Total mass)} x 100
- normality: (equivalents of H+, OH- or e-)/L . N = M x eq/mol, M = N/(eq/mol)
- Arrhenius acid: A material that provides H+ when dissolved in water.
- Arrhenius base: A material which provides OH- when dissolved in water.
- Bronsted Lowry acid: A material which provides H+.
- Bronsted-Lowry base: A material which accepts H+.
- oxidation is the lass of electrons.
- reduction is the gain of electrons.
- neutralization reaction: A reaction between and acid and a base yielding a salt and water.
- precipitation reaction: A reaction in which a solid fall out of solution.
- gas-evolving reaction: A reaction in which a gas bubbles out of solution, leaving the solution.
- addition or synthesis reaction: A reaction in which two or more reactants combine to make one product.
- decomposition reaction: A reaction in which one reactant yields two or more products.
- single displacement reaction: a reaction in which one element displaces another in a compound.
- double displacement or metathesis reaction: A reaction in which all the ions swap partners.
- bonding orbital-a molecular orbital which puts electron density between or above or below the potential bond between two atoms. It is designated by either no superscript or a superscript
- Predict the products for the reaction between the paired elements below and write a balanced equation:
Procedure: First predict the products using single atoms of the elements, and then write a balanced equation using the elemental forms of the elements for the reactants Elemental forms: Al, Li, Mg, O2, N2, F2, P4 and S8<
- O and Li; ion forms Li+ and O2- product is Li2O
balanced equation: 4 Li + O2 6 Li2O
- P and S ;S is more electronegative than P, so P can formally Alose@ all five valence electrons, formal oxidation states would be P5+ and S2- so the product would be P2S5
balanced equation: 4 P4 + 5 S8 6 8 P2S5
- S and F; F is more electronegative than S, so S can formally Alose@ all six valence electrons, formal oxidation state would be S6+ and F- so the product would be SF6
balanced equation: S8 + 24 F2 6 8 SF6
- Al and F ionic compound, ion forms are Al3+ and F- so the product is AlF3
balanced equation: 2 Al + 3 F2 6 2 AlF3
- Mg and N ionic compound, ion forms are Mg2+ and N3- so the product is Mg3N2
balanced equation: 3 Mg + N2 6 Mg3N2
- O and Li; ion forms Li+ and O2- product is Li2O
- 2 Ca + O2------> 2 CaO addition
- CaSO4 + 4 C -----> CaS + 4 CO
- C3H8 + 5 O2 -----> 3 CO2 + 4 H2O special=combustion
- P2O5 + 3 H2O -----> 2 H3PO4 addition
- Na2O + H2O -----> 2 NaOH addition
- 2 C4H10 + 13 O2 -----> 8 CO2 + 10 H2O special=combustion
- 3 Mg(OH)2 + 2 H3PO4 -----> Mg3(PO4)2 + 6 H2O double displacement, special=neutralization
- 2 HCl + Ba(OH)2 -----> BaCl2 + 2 H2O double displacement, special=neutralization
- 2 HgO -----> 2 Hg + O2 decomposition
- 2 AgNO3 + BaCl2 -----> 2 AgCl9 + Ba(NO3)2 double displacement, special=precipitation
- 2 H2O2 -----> 2 H2O + O2 decomposition
- C6H12O6 -----> 6 C + 6 H2O decomposition
- HC2H3O2 + NaOH ------> NaC2H3O2(aq) + H2O double displacement, special=neutralization
- no ionic compounds soluble in water
- CaSO4 is somewhat soluble in water, weak electrolyte
- all gaseous
- H3PO4 soluble in water, weak electrolyte
- NaOH soluble in water, strong electrolyte
- gaseous reaction
- H3PO4 soluble in water( weak electrolyte), Mg(OH)2 and Mg3(PO4)2 insoluble in water
- HCl,Ba(OH)2 and BaCl2 soluble in water, all electrolytes
- no ionic compounds soluble in water present
- AgNO3,BaCl2, and Ba(NO3)2 soluble in water, AgCl insoluble in water
- H2O2, solbule in water, non-electrolyte solution
- C6H12O6, glucose a covalent compund that is soluble in water due to the polar bonds it contains, non-electrolyte solution
- HC2H3O2,NaOH and NaC2H3O2, soluble in water, HC2H3O2 is weak electrolyte, NaOH and NaC2H3O2 are strong electrolytes
- true molecular
- true molecular
- true molecular
- true molecular
- true molecular
- true molecular
- molecular: 3 Mg(OH)2(s) + 2 H3PO4(aq) -----> Mg3(PO4)2(s) + 6 H2O
total ionic: 3 Mg(OH)2(s) + 6 H+(aq) + 2 PO43-(aq) -----> Mg3(PO4)2(s) + 6 H2O
net ionic: 3 Mg(OH)2(s) + 6 H+(aq) + 2 PO43-(aq) -----> Mg3(PO4)2(s) + 6 H2O
- molecular: 2 HCl(aq) + Ba(OH)2(aq) -----> BaCl2(aq) + 2 H2O
tot.ion.: 2 H+(aq) + 2 Cl-(aq) + Ba2+(aq) + 2 OH-(aq) -----> Ba2+(aq) + 2 Cl-(aq) + 2 H2O
net ionic: H+(aq) + OH-(aq) -----> H2O
- true molecular
- molecular: 2 AgNO3(aq) + BaCl2(aq) -----> 2 AgCl(s) + Ba(NO3)(aq)
tot. ion.: 2 Ag+(aq) + 2 NO3-(aq) + Ba2+(aq) + 2 Cl-(aq) -----> 2 AgCl(s) + Ba2+(aq) + 2 NO3-(aq)
net ionic: Ag+(aq) + Cl-(aq) -----> AgCl(s)
- true molecular
- true molecular
- molecular: HC2H3O2(aq) + NaOH(aq) ------> NaC2H3O2(aq) + H2O
tot. ion.: H+(aq) + C2H3O2-(aq) + Na+(aq) + OH-(aq) ------> Na+(aq) + C2H3O2(-aq) + H2O
net ionic: H+(aq) + OH-(aq) ------> H2O
subscript-number in formula indicating atom ratio
coefficient-number in front of a formula indicating ratio of moles or molecules to each other
yeilds arrow-arrow going from reactants to products. Traditionally from left to right
(g)-gaseous
(s)-solid
(l)-liquid
(aq)-aqueous meaning dissolved in water
8
- gas is evolved9
- a solid precipitates from solution- H2SO3
FW = 2(1.008)+32.06+3(16.00) = 82.08 g/mol
%H = {[2(1.008)]/82.08}100 = 2.46%
%S = {32.06/82.08}100 = 39.06%
%O = {[3(16.00)]/82.08}100 = 58.48%
EF = H2SO3
- HClO4
FW = 1.008+35.45+4(16.00) = 100.46 g/mol
%H = {1.008/100.46}100 = 1.00%
%Cl = {35.45/100.46}100 = 35.29%
%O = {[4(16.00)]/100.46}100 = 63.71%
EF = HClO4
- Al(OH)3
FW = 26.98+3(16.00)+3(1.008) = 78.00 g/mol
%Al = {26.98/78.00}100 = 34.59%
%O = {[3(16.00)]/78.00}100 = 61.54%
%H = {[3(1.008)]/78.00}100 = 3.88%
EF = Al(OH)3
- C6H12O6
FW = 6(12.01)+12(1.008)+6(16.00) = 180.16 g/mol
%C = {[6(12.01)/180.16}100 = 40.00%
%H = {[12(1.008)]/180.16}100 = 6.71%
%O = {[6(16.00)]/180.16}100 = 53.29%
EF = CH2O
- NH4OH
FW = 14.01+5(1.008)+16.00 = 35.05 g/mol
%N = {14.01/35.05}100 = 39.97%
%H = {[5(1.008)]/35.05}100 = 14.38%
%O = {16.00/35.05}100 = 45.65%
EF = NH4OH
- C5H5N
FW = 5(12.01)+5(1.008)+14.01 = 79.10 g/mol
%C = {[5(12.01)]/79.10}100 = 75.92%
%H = {[5(1.008)]/79.10}100 = 6.37%
%N = {14.01/79.10}100 = 17.71%
EF = C5H5N
- C12H22O11
FW = 12(12.01)+22(1.008)+11(16.00) = 342.30 g/mol
%C = {[12(12.01)]/342.30}100 = 42.10%
%H = {[22(1.008)]/342.30}100 = 6.48%
%O = {[11(16.00)]/342.30}100 = 51.42%
EF = C12H22O11
- K2Cr2O7
FW = 2(39.10)+2(52.00)+7(16.00) = 294.20 g/mol
%K = {[2(39.10)]/294.20}100 = 26.58%
%Cr = {[2(52.00)]/294.20}100 = 35.35%
%O = {[7(16.00)]/294.2}100 = 38.07%
EF = K2Cr2O7
- CoFe2O4
FW = 58.93+2(55.85)+4(16.00) = 234.63 g/mol
%Co = {58.93/234.63}100 = 25.12%
%Fe = {[2(55.85)]/234.63}100 = 47.61%
%O = {[4(16.00)]/234.63}100 = 27.28%
EF = CoFe2O4
- C - 92.3 %; H - 7.7 %; MW = ~78
C92.3gH7.7g
C92.3g/(12.01g/mol)H7.7g/(1.008g/mol)
C7.685molH7.638mol
C7.685mol/7.638molH7.638mol/7.638mol
C1H1 = EF
EW = 12.01 + 1.008 = 13.02 g/EF
MW/EW = 78/13 = 6 therefore molecular formula is six times the empirical formula of C6H6.
- C - 92.3 %; H - 7.7%; MW = ~104
Same as above, EW the same, MW=104
104/13 = 8 therefore molecular formula is eight times the empirical formula, C8H8.
- C - 93.7 %; H - 6.3 %; MW = ~128
C93.7 gH6.3 g
C93.7g/(12.01 g/mol)H6.3g/(1.008 g/mol)
C7.80molH6.25mol
C7.80mol/6.25molH6.25mol/6.25mol
C1.25H1 can't have fractions of an atom,& since 0.25=1/4 multiply through by 4 to give an empirical formula of C5H4
EW = 5(12.01)+4(1.008) = 64.08 g/EF
MW = 128; MW/EW = 128/64 = 2, therefore MF is twice EF or C10H8.
- Na - 32.4 %; H - 0.71%; P - 21.8 %; O - 45.1 %
Na32.4gH0.71gP21.8gO45.1g
Na32.4g/(22.99g/mol)H0.71g/(1.008g/mol)P21.8g/(30.97g/mol)O45.1g/(16.00g/mol)
Na1.41molH0.704molP0.704molO2.82mol
Na1.41mol/0.704molH0.704mol/0.704molP0.704mol/0.704molO2.82mol/0.704mol
EF = Na2HPO4
- Co - 73.4 %; O - 26.6 %
Co73.4gO26.6g
Co73.4g/(58.93g/mol)O26.6g/(16.00g/mol)
Co1.25molO1.66mol Co1.25mol/1.25molO1.66mol/1.25mol
Co(1 atom)O(1.328 atom) (approximately 1.328 = 1 1/3 therefore multiply through by 3 to give an empirical formula of Co3O4.
- Fe - 36.8 %; S - 21.1 %; O - 42.1 %
Fe36.8gS21.1gO42.1g Fe36.8g/(55.85g/mol)S21.1g/(32.06g/mol)O42.1g/(16.00g/mol)
Fe0.659molS65.8molO2.63mol
Fe0.659mol/0.658molS0.658mol/0.658molO2.63mol/0.658mol
EF = FeSO4
C38.70 gH9.75 gO51.55 g
C38.70 g/(12.01 g/mol)H9.75 g/(1.008 g/mol)O51.55 g/(16.00 g/mol)
C3.222 molH9.673 molO3.222 mol
C3.222 mol/3.222 molH9.673 mol/3.222 molO3.222 mol/3.222 mol
EF = C1H3O1 or CH3O
EW = 12.01 + 3(1.008) + 16.00 = 31.04 g/EF
86/31.04 = 2.77
minimum = 81/31.04 = 2.61
maximum = 91/31.04 = 2.93
May be C3H9O3, data does not fit exactly (Welcome to the real world!!!)
- ruthenium (III) nitrate, Ru(NO3)3
- beryllium iodide, BeI2
- cobalt (III) sulfate, Co2(SO4)3
- nitrous acid, HNO2
- sulfurous acid, H2SO3
- diarsenic trioxide, As2O3
- silver bromide, AgBr
- chloric acid, HClO3
- arsenous acid, H3AsO3
- phosphoric acid, H3PO4
moles propane = 25.0 g/(44.09 g/mol) = 0.567 mol
FW dioxygen = 2(16.00) = 32.00 g/mol
moles dioxygen = 25.0 g/(32.00 g/mol) = 0.781 mol
moles of O2 required to react completely with o.567 mol of
C3H8 = (5/1)(0.567 mol) = 2.835 mol of O2
There are only 0.781 mol of O2 available, thus the limiting reactant is O2.
Theoretical yield of CO2 = (3/5)(mol of limiting reactant)
= (3/5)(0.781 mol) = 0.469 mol
FW CO2 = 12.01 + 2(16.00) = 44.01 g/mol
grams CO2 produced theoretically = (44.01 g/mol)(0.469 mol)
= 20.6 g
moles CoSO4 = 19.50 g/(155.00 g/mol) = 0.1258 mol
FW NaOH = 22.99+16.00+1.008 = 40.00 g/mol
moles NaOH = 8.50 g/(40.00 g/mol) = 0.2125 mol
mol of NaOH required to react with 0.1258 mol of CoSO4 is equal to (2/1)(0.1258 mol) = 0.2516 mol of NaOH required
There is only 0.2125 mol of NaOH, thus the NaOH will be all used up, and is the limiting reactant.
Theoretical yield of both Co(OH)2 and Na2SO4 in moles:
(2)(0.2125 mol) = 0.1063 mol
FW Co(OH)2 = 58.94+2(16.00)+2(1.008) = 92.96 g/mol
Actual yield in mol = 9.00g/(92.96 g/mol) = 0.0968 mol
%Yield = [(0.0968 mol)/(0.1063 mol)]x100 = 91.1%
OR
FW Na2SO4 = 2(22.99)+32.06+4(16.00) = 142.04 g/mol
Actual yield in mol = 13.75 g/(142.04 g/mol) = 0.0968 mol
%Yield = [(0.0968 mol)/ (0.1063 mol)]x100 = 91.1%
FW Fe(NO3)2 = 55.85+2(14.01)+6(16.00) = 179.87 g/mol
moles Fe(NO3)2 = 20.21 g/(179.87 g/mol) = 0.1124 mol
FW Mg(OH)2 = 24.31+2(16.00)+2(1.008) = 58.33 g/mol
moles Mg(OH)2 = 5.93 g/(58.33 g/mol) = 0.1017 mol
All ratios are 1:1. The lesser amount of moles is that of Mg(OH)2 so it is the limiting reactant. The amount of moles that theoretically can be produced for both Fe(OH)2 and Mg(NO3)2 is equal to (1/1)(0.1017 mol) = 0.1017 mol
FW Fe(OH)2 = 55.85+2(16.00)+2(1.008) = 89.87 g/mol
Actual yield of Fe(OH)2 = 8.82 g/(89.87g/mol) = 0.0981 mol
%Yield = [0.0981 mol/(0.1017 mol)]x100 = 96.5%
OR
FW Mg(NO3)2 = 24.31+2(14.01)+6(16.00) = 148.33 g/mol
actual yield Mg(NO3)2 =14.56/(148.33 g/mol)= 0.0982 mol
%Yield = [0.0982/(0.1017 mol)]x100 = 96.6%
- FW Li2O2 = 2(6.940)+2(16.00)= 45.88 g/mol
mol = 29.0 g/(45.88 g/mol) = 0.6321 mol
- FW U3O8 = 3(238.07)+8(16.00) = 842.21 g/mol
mol = 576.01 g/(842.21 g/mol) = 0.68393 mol
- FW Fe(OH)3 = 55.85+3(16.00)+3(1.008) = 106.87 g/mol
mol = 92.3 g/(106.87 g/mol) = 0.864 mol
- FW TiO2 = 47.90+2(16.00)= 79.90 g/mol
6.17 g/(79.90 g/mol) = 0.772 mol
- FW Co2O3 = 58.94+3(16.00) = 107.02 g/mol
11.9 g/(107.02 g/mol) = 0.111 mol
- FW H2S = 2(1.008)+32.06=34.08 g/mol
g = (34.08 g/mol)(6.93 mol) = 236.17 g
- FW XeF4 = 131.30+4(19.00)= 207.30 g/mol
(207.30 g/mol)(0.00655 mol)=1.358 g
- FW O2 = 2(16.00)=32.00 g/mol
(32.00 g/mol)(6.98 mol) = 223.36 g
- FW U3O8 = 3(238.07)+8(16.00)= 842.21 g/mol
(7.05 mol)(842.21 g/mol)= 5938 g
- FW ICl4 = 126.94+4(35.45)= 268.74 g/mol
(0.1973 mol)(268.74 g/mol)=53.02 g
- Na2O basic anyhydride
- SO3 acidic anhydride
- P2O5 acidic anhydride
- MgO basic anhydride
- Cl2O7 acid anhydride
- 3.998 g of LiOH dissolved in 15.00 mL of water
FW LiOH = 6.94 + 16.00 + 1.008 = 23.95 g/mol
moles LiOH = 3.998 g/23.95 g/mol = 0.1669 mol
molarity = 0.1669 mol/0.01500 L = 11.27 M
- 59.99 g of LiCl dissolved in 135.88 mL of water
FW LiCl = 6.94+35.45 = 42.39 g/mol
moles LiCl = 59.99 g/42.39 g/mol = 1.415 mol
molarity = 1.415 mol/0.13588 L = 10.41 M
- 20.00 g of Na3PO4 dissolved in 50.00 mL of water
FW Na3PO4 = 3(22.99)+30.97+4(16.00) = 163.94 g/mol
moles Na3PO4 = 20.00 g/163.94 g/mol = 0.1220 mol
molarity = 0.1220 mol/0.05000 L = 2.440 M
- 55.00 g of H2SO4 dissolved in 35.00 mL of water
HW H2SO4 = 2(1.008) + 32.06 + 4(16.00) = 98.08 g/mol
moles H2SO4 = 55.00 g/98.08 g/mol = 0.5608 mol
molarity = 0.5608 mol/0.05500 L = 10.20 M
- 100.00 g of C12H22O11 dissolved in 100.00 g of water
FW C12H22O11 = 12(12.01) + 22(1.008) + 11(16.00) = 342.30 g/mol
moles C12H22O11 = 100.00 g/342.30 g/mol = 0.2921 mol
molarity = 0.2921 mol/0.10000 L = 2.921 L
NAVA = NBVB
NA = NBVB/VA
1.225 M NaOH (1 eq/mol) = 1.225 N NaOH
NA = NBVB/VA = (1.225 N)(26.97 mL)/(20.00 mL) = 1.652 N H2SO4
(1.652 N H2SO4)/(2 eq/mol) = 0.8260 M H2SO4
NAVA = NBVB
NB = NAVA/VB
(5.553 M HCl)(1 eq/mol) = 5.553 N HCl
NB = NAVA
/VB = (5.553 )(22.33 mL)/(15.00 mL) = 8.267 N
(8.267 N)/(1 eq/mol) = 8.267 M LiOH
- Fe(C2H3O2)2 soluble
- PbSO4 insoluble
- NH4NO3 soluble
- AgCl insoluble
- MnSO4 soluble
- Fe(OH)2 insoluble
- KOH soluble
- Zn3(PO4)2 insoluble
- Na3PO4 soluble
- CaCO3 insoluble
M1V1 = M2V2
M1V1/M2 = V2 = (18.00 M)(85.00 mL)/(3.5565 M) = 430.2 mL
M1V1 = M2V2
M2V2/M1 = V1 = (2.450 M)(3.500 L)/(12.00 M) = 0.7146 L or 714.6 mL