STUDY SHEET FOR CHEM 101 TEST 3 (DELANEY)
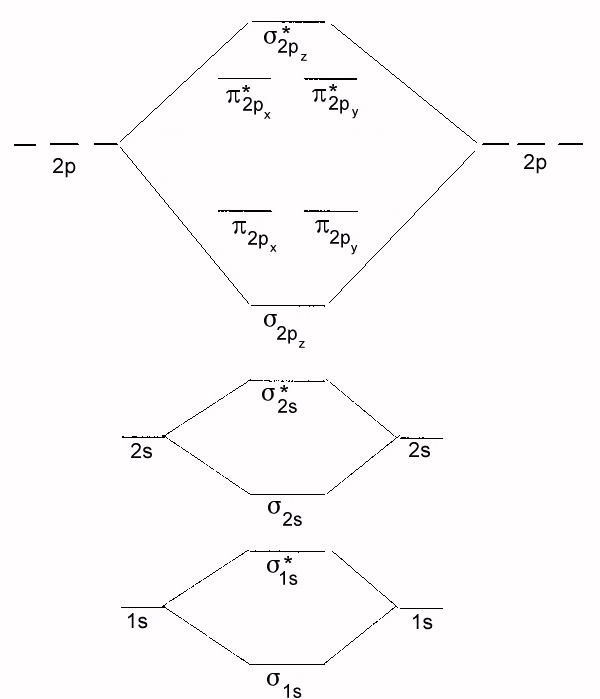
- Using the molecular orbital scheme above for diatomic species predict the bond order for the
following proposed diatomic species.
- FN2+
- F2
- C2
- BBe
- O2-
- N2
- BF2-
- CN-
- NO
- CN-
Are the species above diamagnetic or paramagnetic?
- For each of the compounds below, draw the Lewis structure; then apply VSEPR theory to predict the
molecular structure; name the molecular structure type; tell which hybrid orbitals would be used and
finally, draw the structure.
- PBr3
- CCl4
- CO2
- BF3
- PF5
- SF4
- SeCl6
- I3-
- XeF4
- ICl3
- ClO3-
- Properly name the following compounds.
- MnSO4
- AlPO4
- P2O3
- K3PO4
- KClO3
- Sc2S3
- N2O5
- Ca(NO3)2
- CoNO3
- FeSO4
- What is the rationale that allows VSEPR theory to predict molecular structure?
- What is the rationale that allows valence bond theory (hybrid orbital theory) to explain covalent bonding?
- What similar rationale to that used for valence bond theory that allows molecular orbital theory to explain covalent bonding?
- Define:
- bonding orbital
- anti-bonding orbital
- molarity
- percent by mass
- normality
- Arrhenius acid
- Arrhenius base
- Bronsted-Lowry acid
- Bronsted-Lowry base
- oxidation
- reduction
- neutralization reaction
- precipitation reaction
- gas-evolving reaction
- addition or synthesis reaction
- decomposition reactioin
- single displacement reaction
- double displacement or metathesis reaction
- Predict the products for the reaction between the paired elements below and write a balanced equation:
- O and Li
- P and S
- S and F
- Al and F
- Mg and N
- Balance the reactions below by inspection:
- _____ Ca(s) + _____ O2(g) ---------> _____ CaO(s)
- _____ CaSO4(s) + _____ C(s)-----> ____ CaS(s) + _____ CO(g)
- _____ C3H8(g) + _____ O2(g) -------> _____ CO2(g) + _____ H2O(g)
- _____ P2O5(s) + _____ H2O(l) -------> ______ H3PO4(aq)
- _____ Na2O(s) + ______ H2O(l) -----> ______ NaOH(aq)
- _____ C4H10(g) + ______ O2(g) ------> _____ CO2(g) + _____ H2O(l)
- ___ Mg(OH)2(s) + ___H3PO4(aq) -----> ____ Mg3(PO4)2(s) + ____ H2O(l)
- ___ HCl(aq) + ___ Ba(OH)2(aq) ----> ___ BaCl2(aq) + ____ H2O(l)
- ____ HgO(s) -----> _____ Hg(l) + _____ O2(g)
- __ AgNO3(aq) + __ BaCl2(aq) ---> ___ AgCl(s) + ___ Ba(NO3)2(aq)
- _____ H2O2(aq) ------> H2O(l) + O2(g)
- _____ C6H12O6(s) -------> _____ C(s) + _____ H2O(g)
- ___ HC2H3O2(aq) + ___ NaOH(aq) -----> ___ NaC2H3O2(aq) + ___ H2O(l)
- For each of the equations above after it is balanced decide whether it is an addition reaction, a decomposition reaction, single displacement reaction or a double displacement (metathesis) reaction.
- For each of the equations above in problem 11, decide whether they are any of the special types of reactions such as combustion, oxidation-reduction, neutralization, pecipitation or diproportionation reactions.
- For each of the products and reactants above in problem 11, decide whether they are likely to be soluble in water. Then decide whether the soluble materials are likely to be strong, weak or non-electrolytes.
- For each of the equations above in problem 11, decide whether the equation is a molecular or ionic equation. If it is an ionic equation, write the total ionic equation and then the net ionic equation. Which ions end up being spectator ions?
- Define subscript, coefficient, yields arrow, (g), (s), (l), (aq), Δ, 8 and 9.
- What is implicitly implied in a balanced chemical equation?
- Classify the reactions in problem 11 as addition (synthesis), decomosition, single displacement, double displacement.
- Provide the formula weight and the weight percentage of each element in the compounds below. What is the empirical formula for each?
- H2SO3
- HClO4
- Al(OH)3
- C6H12O6
- NH4OH
- C5H5N
- C12H22O11
- K2Cr2O7
- CoFe2O4
- Find the empirical formulae of the compounds from the weight percentages given below. Find the empirical weight. Where appropriate, find the molecular formula.
- C - 92.3 %; H - 7.7 %; MW = ~78
- C - 92.3 %; H - 7.7%; MW = ~104
- C - 93.7 %; H - 6.3 %; MW = ~128
- Na - 32.4 %; H - 0.71%; P - 21.8 %; O - 45.1 %
- Co - 73.4 %; O - 26.6 %
- Fe - 36.8 %; S - 21.1 %; O - 42.1 %
- A compound is 38.70 % carbon, 9.75 % hydrogen and 51.55 % oxygen. What is the empirical formula of the compound?
- What is the empirical weight of the compound in problem 21?
- If the approximate molecular weight of the compound in problem 21 is 92 + 5 g/mol , what is the molecular formula of the compound ?
- Write the correct formula from the name
- rutheniumm (III) nitrate
- beryllium iodide
- cobalt (III) sulfate
- nitrous acid
- sulfurous acid
- diarsenic trioxide
- silver bromide
- chloric acid
- arsenous acid
- phosphoric acid
- What is the limiting reagent when 25.0 g of propane is burned with 25.0 g of dioxygen (O2)? How much CO2 is produced (in grams)?
C3H8(g) + 5 O2(g) -----------------> 3 CO2(g) + 4 H2O(g)
- When 19.50 g of cobalt (II) sulfate was reacted with 8.50 g of sodium hydroxide the products produced were 9.00 g of colbalt (II) hydroxide and 13.75 g of sodium sulfate. What was the limiting reactant? What was the percent yield? The balanced equation is given below:
CoSO4 + 2 NaOH ------------> Co(OH)2 + Na2SO4
- An experiment is performed in which 20.21 g of iron (II) nitrate is reacted with 5.93 g of magnesium hydroxide to yield 8.82 g of iron (II) hydroxide and 14.56 g of magnesium nitrate.
- Write a balanced equation for the reaction
- What is the limiting reagent?
- What is the per cent yield?
- How many moles are there in the masses of the substances indicated below?
- 29.0 g of Li2O2
- 576.01 g of U3O8
- 92.3 g of Fe(OH)3
- 6.17 g of TiO2
- 11.9 g of Co2O3
- What masses are present in the amount of moles of the substances below?
- 6.93 moles of H2S
- 0.00655 moles of XeF4
- 6.98 moles of O2
- 7.05 moles of U3O8
- 0.1973 moles of ICl4
- Are the materials below acid anhydrides or basic anhydrides?
- Na2O
- SO3
- P2O5
- MgO
- Cl2O7
- What is the molarity of the solutions below?
- 3.998 g of LiOH dissolved in 15.00 mL of water
- 59.99 g of LiCl dissolved in 135.88 mL of water
- 20.00 g of Na3PO4 dissolved in 50.00 mL of water
- 55.00 g of H2SO4 dissolved in 35.00 mL of water
- 100.00 g of C12H22O11 dissolved in 100.00 g of water
- What is the molarity of an H2SO4 solution if a 20.00 mL sample of that solution is titrated with 26.97 mL of 1.225 M NaOH?
- What is the molarity of an LiOH solution if a 15.00 mL sample of the solution is titrated with 22.33 mL of 5.553 M HCl solution?
- Are the compounds below soluble in water?
- Fe(C2H3O2)2
- PbSO4
- NH4NO3
- AgCl
- MnSO4
- Fe(OH)2
- KOH
- Zn3(PO4)2
- Na3PO4
- CaCO3
- To what volume must 85.00 mL of 18.00 M H2SO4 be diltued to yield a solution that is 3.5565 M H2SO4?
- What volume of 12.00 M HCl must you start with to successfully dilute it to 3.500 L of 2.450 M HCl?

