CARBOCATIONS DERIVED FROM DEAMINATION AS INITIATORS
FOR CATIONIC POLYMERIZATIONS
Ron Darbeau*, Daneisha Davis**, Mark Delaney, Delana
Gbenekama**, Kevin James and Ulku Ramelow

A Contribution from the
Department of Chemistry
P. O. Box 90455
McNeese State University
Lake Charles, LA 70609
Cationic polymerizations initiated via carbocations have usually relied upon carbocations generated via a organic halide and a Friedel-Crafts type catalyst. Several problems are inherent in this method. First, the "carbocation" is almost certainly not "free" but instead is most likely either involved with a complex or a tight ion-pair with the Friedel-Crafts catalyst. Second, the solubility of such systems is limited. Third, it is difficult to quantitate the amount of "carbocation" produced. The production of carbocations via deamination of a carboxylamide or sulfonamide after nitrosation to form the unstable nitrosamide (the precursor to the carbocation) has several advantages. The amide is nitrosated using dinitrogen tetroxide to yield the unstable nitrosamide. The nitrosamide undergoes rearrangement and eventual thermal decomposition yielding the carbocation, dinitrogen, and a carboxylate ion. The carbocation and the carboxylate ion are separated from each other by the dinitrogen. This grouping of ions is termed a nitrogen (or nitrogenous) separated ion pair (NSIP). The separation of the carbocation from the carboxylate by the dinitrogen allows the carbocation to react with nucleophiles other than the carboxylate ion. The process for forming the NSIP is demonstrated below using N-benzylpivalamide.

The advantages of carbocations generated via deamination are several:
Carbocations generated via deamination have been used to polymerize or oligomerize monomers with the following results:
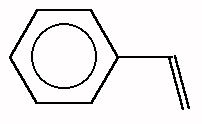 Styrene: Freshly distilled styrene in dry hexane was polymerized
though decomposition of N-benzyl-N-nitrosopivalamide at 40oC
for 3 hours. The resultant yellow solution was quenched with 2-propanol. The product polystyrene was precipitated in methanol. The yield was a
conversion of 16.5 % styrene to high polymer. The viscosity average molecular mass
was 1.25 x 106. The melt temperature of the polymer was 165oC. Tests (including
NMR) are now underway to determine if the high melt temperature is due to a high
content of syndiotactic polystyrene or due to cross-linking, which would also explain the
very high molecular mass. (R. W. Darbeau, M. S. Delaney, U. Ramelow, and K. R.
James, Organic Letters, 1(5), 761 (1999)).
Styrene: Freshly distilled styrene in dry hexane was polymerized
though decomposition of N-benzyl-N-nitrosopivalamide at 40oC
for 3 hours. The resultant yellow solution was quenched with 2-propanol. The product polystyrene was precipitated in methanol. The yield was a
conversion of 16.5 % styrene to high polymer. The viscosity average molecular mass
was 1.25 x 106. The melt temperature of the polymer was 165oC. Tests (including
NMR) are now underway to determine if the high melt temperature is due to a high
content of syndiotactic polystyrene or due to cross-linking, which would also explain the
very high molecular mass. (R. W. Darbeau, M. S. Delaney, U. Ramelow, and K. R.
James, Organic Letters, 1(5), 761 (1999)).
 Cyclohexene: Neat cyclohexene was polymerized using
N-benzyl-N-nitrosopivalamide as initiator at 40oC and allowed to
continue for 3 hours. After three hours, the reaction was
quenched with 2-propanol and unreacted cyclohexene allowed
to evaporate from the reaction mixture. The result was a high
polymer with a waxy feel reminiscent of polyolefins. Two possible structures are shown
below:
Cyclohexene: Neat cyclohexene was polymerized using
N-benzyl-N-nitrosopivalamide as initiator at 40oC and allowed to
continue for 3 hours. After three hours, the reaction was
quenched with 2-propanol and unreacted cyclohexene allowed
to evaporate from the reaction mixture. The result was a high
polymer with a waxy feel reminiscent of polyolefins. Two possible structures are shown
below:
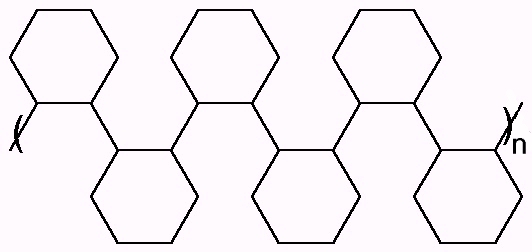
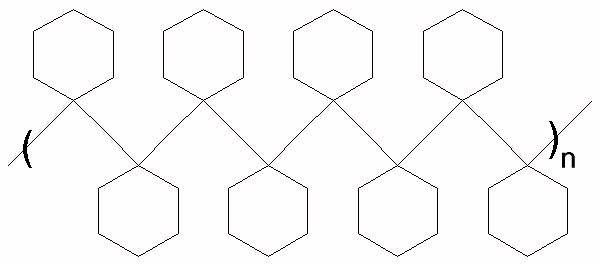
This structure results from a regular polymerization across the double bond of the cyclohexene. The structure below would result from a rearrangement of the carbocation to a tertiary carbocation.
 Isobutylene (2-methylpropene): Isobutylene was oligomerized at
25oC at 28 psig in dichloromethane using N-benzyl-N-nitrosopivalamide as initiator. The reaction was allowed to
proceed for 3 hours. The reaction was quenched with 2-propanol and the unreacted isobutylene and the dichloromethane solvent allowed to
evaporate from the reaction mixture. A small yield of gummy oligomer resulted from
this procedure.
Isobutylene (2-methylpropene): Isobutylene was oligomerized at
25oC at 28 psig in dichloromethane using N-benzyl-N-nitrosopivalamide as initiator. The reaction was allowed to
proceed for 3 hours. The reaction was quenched with 2-propanol and the unreacted isobutylene and the dichloromethane solvent allowed to
evaporate from the reaction mixture. A small yield of gummy oligomer resulted from
this procedure.
NOTES ON POLYMERIZATIONS
It must be emphasized that none of the polymerizations described here have been optimized. The current and most recent research has been to define the scope of the ability of deaminatively generated carbocations to initiate cationic polymerizations. Further study of this class of initiators is continuing. Polymerizations were done either in glassware, when appropriate, or in a 300 mL 316 stainless steel Parr high pressure reactor, when appropriate.
MOLECULAR MODELING
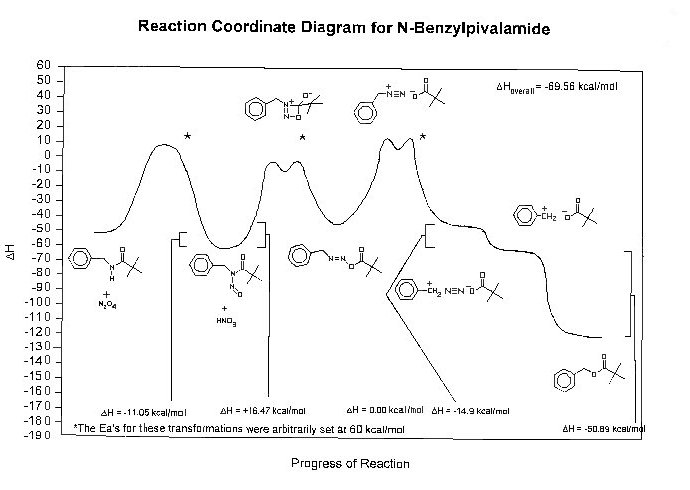
A molecular modeling study was performed to help further the understanding of the process of the formation of carbocations via deamination. Many structures were drawn and not all have yet been minimized. Reaction coordinate diagrams for N-t-butylpivalamide, -sec-butylpivalamide, N-benzylpivalamide and N-benzyl-phenylsulfonamide have been prepared. For this poster only the reaction coordinate diagrams for N-benzylpivalamide and N-benzylphenylsulfonamide are displayed.
Procedure: Molecules were drawn using ChemSketch 4.5 (ACD Labs) , cleaned and optimized for 3D configuration. Each model was then saved as a *.sk2 file and exported as a mol (*.mol) file. The mol (*.mol) file for each molecule was then imported into PCModel (Serena Software) and the structure minimized. The Hf, strain energy and MMX energy were recorded for each structure. An approximate reaction coordinate diagram was developed with this information for each deamination series and graphed using SigmaPlot (Jandel Scientific).
Results and Discussion: Actual experiments of deamination have not been performed for N-t-butyl-2-methylpropanamide and N-t-butylphenylsulfonamide. Previous experiments and steric studies have indicated a steric interference problem with the nitrosation of N-t-butylpivalamide and N-sec-butylpivalamide. Deamination experiments with N-benzylpivalamide and N-benzylphenylsulfonamide are routinely done successfully. The carbocations generated from the sulfonamides are normally considered to react to greater extent with nucleophiles other than the counteranion (sulfonate) when compared to carbocations generated from carboxamides (i.e. pivalmides). This difference in reactivity is normally explained by invoking the greater nucleophilicity of carboxylate ion (i.e. pivalate ion) when compared to the sulfonate ion. If, however, the last excited state before collapse of NSIP to the ester is considered to be the ion pair of the carbocation and the anion after expulsion of dinitrogen from the NSIP, there may also be a thermodynamic explanation for the difference in reactivities. The sulfonamide generated NSIPs have a very high endothermic barrier to reaction in that case, while the pivalamide generated NSIPs show a very slightly endothermic (to exothermic in some cases) process. Thus NSIPs generated to sulfonamides should be more accessible to nucleophiles other than the sulfonate. Further work will include completion of the molecular modeling studies and NMR studies to determine the overall activation energy for the process. Molecular modeling studies in which the NSIPs are reacted with various nucleophiles are also planned.
ACKNOWLEDGMENTS
This research was supported by the Shearman Research Initiative Fund and the Department of Chemistry, McNeese State University. Ron W. Darbeau* would like to thank the Calcasieu Parish Industrial & Development Board Endowed Professorship for Support. Daneisha Davis** and Delana Gbenekama would like to thank Project SEED 2000 at McNeese for support.
This poster is available in downloadable WordPerfect 9 format, in downloadable pdf (Adobe Acrobat) format, in downloadable ppt (PowerPoint) format and in html at:
https://chemprof.tripod.com/poster.htm